The Food and Drug Administration (FDA) is on a mission for new legislation allowing patients to acquire hearing aids over the counter without a prescription that will increase accessibility for millions.
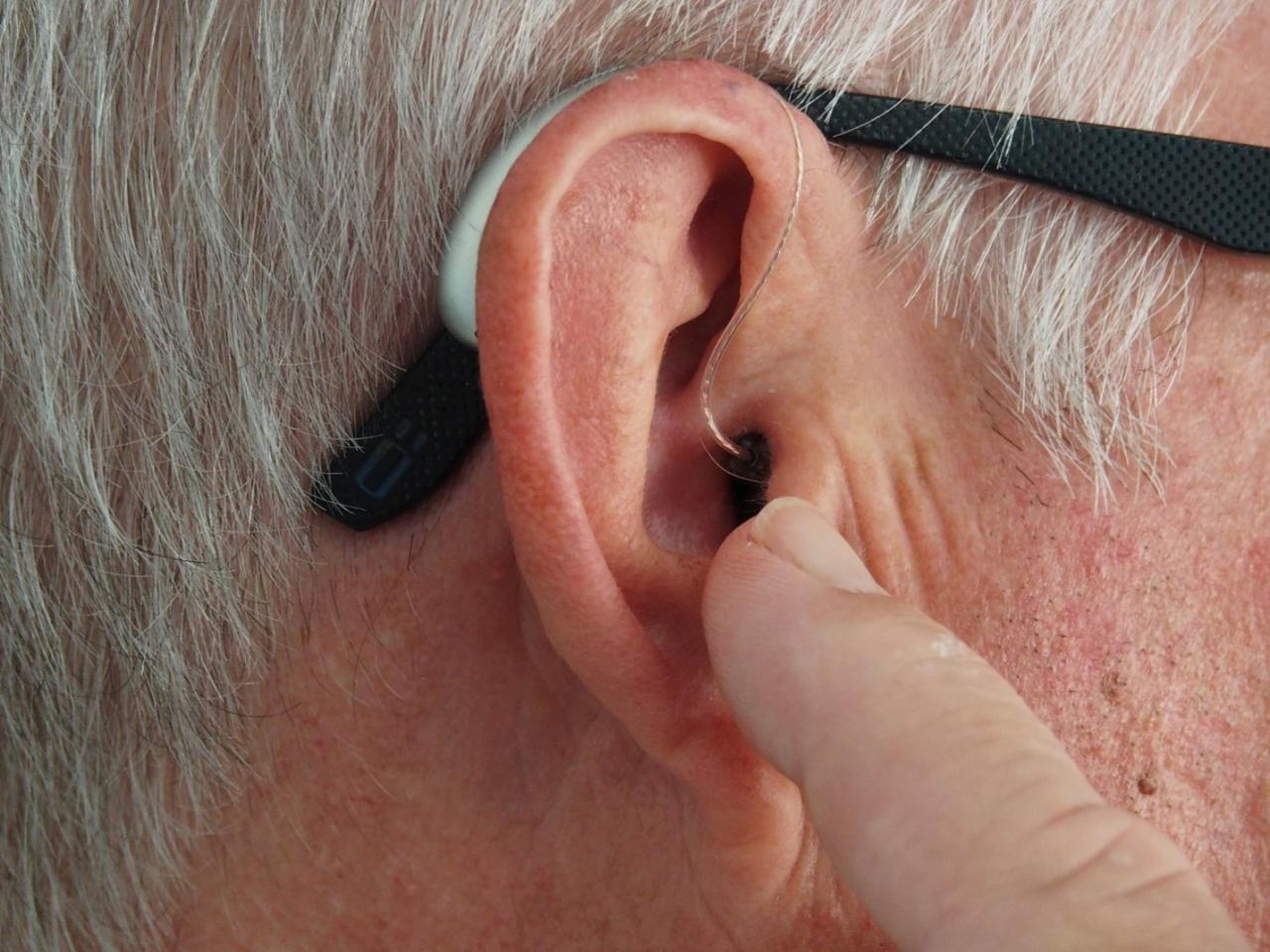
The Food and Drug Administration wants to make hearing aids more accessible and plans on changing regulations so people can get an hearing aid without requiring a prescription. The FDA disclosed that an estimated 37.5 million American adults experience difficulty hearing with patients seeking to get a hearing aid to require patients for a medical examination or special fitting.
This move by the FDA will increase the accessibility and affordability of hearing aids for millions of Americans that experience mild to moderate hearing loss. The FDA proposes changing the rules to offer hearing aids as over-the-counter (OTC) medical devices that are expected to be finalized by early 2022 permitting people to buy them in stores or online without a prescription.
The FDA is limiting OTC hearing aids that aren’t intended for children under the age of 18 or adults with severe hearing loss with these cases commonly requiring specialized hearing aids. The FDA is receiving support in this new ruling from medical organizations across the country including The Hearing Loss Association of America.
This ruling will give more people access to an OTC medical device with the hopes of encouraging adults to take the first steps toward good health. This might seem like a new development but there is a big push for OTC hearing aids since 2017 when Senator Elizabeth Warren introduced a bipartisan Over-the-Counter Hearing Aid Act with the same legislators praising the FDA.
The FDA Reauthorization Act in 2017 signed the OTC hearing aid legislation into law stating the FDA “must categorize certain hearing aids as over-the-counter hearing aids and issue regulations regarding those hearing aids.” Though the legislation was signed into law,it was followed by four years of public inaction until Biden took office.
President Biden included OTC hearing aids in the executive order on “Promoting Competition in the American Economy” singling out hearing aids being an overly high-priced necessity resulting in more looking to the FDA to take immediate action. The FDA has regulated hearing aids as prescription medical devices for decades, in an arrangement to add to the effort and costs people must expand to get them.
The new proposed law would change this agreement and the FDA reassures that people who have limited access will have fewer requirements to have access to the essential piece of medical equipment. From the market perspective, this will elevate competition while putting regulators under scrutiny that aren’t approved to sell hearing aids but are effectively using a loophole for this rule marketing “personal and amplification products”(PSAPs).
A PSAP seems similar to in design and performance of a hearing aid but has an edge on the competition being a product that can be sold straight to consumers without needing a prescription. These devices areal so built for people with normal hearing but want to increase the volume that is different than a hearing aid.
Despite having an alternative to hearing aids that are easy to access, it is not the same device needed for people with impaired hearing. This legislation will help millions of Americans with hearing problems that is more affordable and accessible.